
Thermal Reduction
Recovery 2.0 utilizes heat to perform the Thermal Reduction process to vaporize
waste water
and
solid waste
materials into a gaseous state and a recovered solid fraction.
This type of waste decomposition is a method that allows the recovery of basic molecular elements.
The waste vaporization process involves a phase change in the
feedstocks
and the conversion into the various
output products.
Recovery 2.0, uses a pyrolysis system as a processing method designed NOT to release open emissions but rather operates on a
mass balance equilibrium
basis where the output products are captured and recovered.
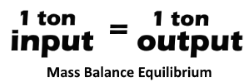
The Process
Recovery 2.0 involves a Two Step thermal reduction
process,
the Heating Stage and the Pyrolysis Stage.
The heating stage harnesses a primary
heat source
in a separate reverberatory chamber and transfers that heat into the pyrolysis chamber.
The pyrolysis stage is performed in an oxygen starved (restricted air free flow) environment
designed to prevent combustion and the production of unwanted combustion by-products.
Heat in the pyrolysis chamber is elevated past the point of vaporization of the waste feedstocks
which are captured directly into the Recovery 2.0
working fluids
pipelines.
The pyrolysis system
Outputs
are largely determined by the pressure, temperature and dwell time within the pyrolysis chamber and
are captured in order to achieve or maintain a
mass balance
equilibrium.
Any primary heat stage emissions that may be generated are contained in a separate pathway and recovered or regenerated.
Flexibility
The working concept behind the Recovery 2.0 system is to design a functioning process that provides flexible options as to the
pathways,
incoming waste
feedstocks
and product
outputs
and to provide the ability to swap from one to another as seamlessly as possible.
In regard to the choice of primary energy
sources
the design needs to accommodate intermittent alternative energy sources
while maintaining reliable and redundant production.
The flexibility to operate or switch between multiple sources that may run on a side by side, comparative basis,
allows for an innovative and comparative environment to advance and develop improved methods and technologies.
Operating multiple independent Thermal Reduction Units simultaneously provides a unique opportunity
to create unprecedented symbiotic efficiencies.
Primary Energy Sources
used to drive the
Thermal Reduction of Waste
| Heat Source | Description |
| Solar | Concentrated Thermal Solar |
| Electricity | Electric Resistance Heating |
| Microwave | Focused Molecular Stimulation |
| Plasma Arc Energy | Ionic Plasma Vaporization |
| Oxy-Combustion |
Hyrdogen, Carbon or
Hydrocarbon Fuels |
|
Exothermic
Energy Extraction |
REDOX Reactions
- Oxidation & Displacement |
| Other |
Recovered, Harvested or
other Alternative Energy |
Heat Sources
Options for sources of heat range from combustion of
fossil fuels
or
BioEnergy Renewables
with emission capture.
The use of solid recovered carbon as a green coal substitute may be an ideal use case as a heat source.
Exploration of carbon free heat sources may be a desirable option with the use of
Alternative Energy
such as clean
Electricity,
Hydrogen
or
Concentrated Solar
or more exotic options including
Plasma Arc Energy.
Whichever primary energy source is chosen the Recovery 2.0 system relies on a
Multi-Stage
energy recovery process.
An insulating
envelope
surrounding the thermal reduction units may maximize heat recovery potential.
Concentrated Solar
Wherever possible and geographically feasible the use of
Concentrated Solar
energy as a source to generate a high heat sufficient
to drive the thermal reduction process makes sense.
Thermal solar energy may be focused onto a receiver that is designed to convert a
thermal media into a high temperature heat transfer source adequate to preform the pyrolysis process within the
thermal reduction chamber.
Any excess heat that is generated may be sent for thermal energy storage or may be used to generate
electricity.
There are several opportunities to extract heat or electricity by
Light Energy
Harvesting.
Electricity
Electric Heat, Radiant or Induction Heat (from external green electricity sources)
may be sourced on demand.
Electricity as a heat source has flexible variable temperature control.
Electricity
may be tapped into from the internal
Energy Management
Control System and dispatched as prioritized.
Microwave
The thermal reduction of waste materials using microwave energy may be an efficient pyrolysis process
since you heat from the inside out.
There is no need to heat the outer shell infrastructure of the furnace.
Focusing direct heat on the waste feedstock will quickly pyrolysis the materials into
a vaporized
Hot Gas
and a solid reside fraction.
Different waste feedstocks may be more or less receptive to Microwave Stimulation requiring the aid of a catalyst
to enhance the Focused Molecular Stimulation.
In some cases the blending of wastestreams may achieve the same result to replace the catalyst.
In the early 1990s we would typically dose the incoming feedstock with recovered pyrolysis oil to
kick start the speed of the reaction time.
Plasma Arc Energy
One interesting source of heat to drive the thermal reduction process is
Plasma Arc Energy.
Plasma Arc generates extreme high temperatures that can be focused on targeted localized areas.
An electric arc causes an Ionic Plasma reaction in the immediate surrounding environment
(typically air or some other pre-selected gas) which results in emitting heat in the order of several thousand degrees °C.
Typical Plasma Arc systems may not be a two stage design, the electrode arc or plasma torch are commonly applied directly
within the pyrolysis chamber.
The extreme high energy and heat causes the waste materials contained within the pyrolysis chamber (or gasification furnace)
to instantaneously vaporize.
This high temperature Vaporization may require some additional cleaning and refining steps if the plasma arc
draws in air from the ambient atmosphere. Also the electricity source used to generate the Plasma Arc Heat is a critical factor
in this method of thermal reduction.
Oxy-Combustion
The use of an
Oxy Combustion
processes combine with emission confinement and regeneration systems are a viable strategic method of
driving a Thermal reduction operation with sustainable fuels.
Oxy-Combustion Fuels -
Hydrogen,
Carbon
and
Hydrocarbons
Hydrogen
Hydrogen Recovery Yields
The use of
Hydrogen
as a primary energy source to begin the thermal reduction process is made possible by
Hydrocarbon Splitting.
Harnessing Hydrogen energy as a fuel to heat a thermal reduction process provides an opportunity to
evolve methods of combining carbon and hydrogen in the absence of oxygen to produce
the required heat and a hydrocarbon output. This output may be cycled for the regeneration of clean hydrogen.
Recovered Solid Carbon
Recovered Solid Carbon represents the largest share volume of
recovery yields
from mixed wastestreams.
The Oxy-Combustion of
Recovered Carbon
provides a huge opportunity to develop a sustainable economy, based on an environmentally contained,
centralized or decentralized
regeneration cycle
for waste treatment.
Hydrocarbon Fuels
The recovery of materials form a mix of various wastestreams results in a wide
range of results
depending on the contents of each individual wastestream and its source.
The trends tend to be somewhat consistent for the types of wastestreams generated from the same sources.
Since most mixed Municipal Solid Waste (MSW) streams contain mostly a variety of Plastics, Paper, food and organic wastes,
which at its fundamental or elemental root are just a mix of Carbon, Hydrogen and Oxygen.
This group of basic building block materials, all together are classified as types of
hydrocarbons.
The recovery of these hydrocarbon materials provide an opportunity to develop a range of intermediate products
that may be used as
sustainable fuels
to replace traditional Fossil Fuels
Exothermic Energy Extraction
The use of
Exothermic Energy
Extraction is a method of harvesting heat to drive an neighboring endothermic process
Exothermic heat is generated from REDOX Reactions as elemental bonds are being formed.
Our particular interest is focused around the
Oxidation & Displacement
process that occurs when refining metal oxides.
A similar effect may be harvested from the
Hydrometallurgy
process of dissolving Metals in acid solutions in the Recovery procedure.
The process of forming
Hydroxides
also provides an opportunity to Extract Exothermic heat
during the recovery of minerals and salts.
Alternative Energy
A variety of
Alternative Energy
sources and
BioEnergy Renewables
are a viable choice.
The choice of a select few fuel sources to provide the primary heat for the thermal reduction process
may provide a unique solution through oxidation or controlled combustion.
The oxidation emissions may be channeled into a closed pipeline and used as the working fluid and recovered
into valorized products.
A
Solid Carbon/CO2
Recovery Cycle may be ideal for this application, In addition
Syngas
may also be an option.
Fossil Fuels
The option to use traditional
Fossil Fuels
exists with a focus on the responsibility for the avoidance of combustion emissions.
Current efforts have shifted towards the potential use of what is referred to as Green Fuels such as Syngas
(SNG),
Renewable Natural Gas
(RNG)
or Methane (CH4).
In the Methanation process, also known as Power to Gas, Carbon Dioxide (CO2) and Hydrogen (H2) is converted into Methane (CH4).
This is used as a direct substitute for traditional Natural Gas.
Conversion into Liquid Fuels such as Naphtha, Methanol and Ethanol are an expanding area of interest particularly in regards to
the production of Transportation Fuels like Gasoline, Diesel and Aviation fuel.
Input Feedstocks
Typical raw inputs of
wastestream feedstocks
in the Recovery 2.0 thermal reduction process fall into two main classifications,
liquids or solids.
The main volumes of liquids are in the form of
Brine & Waste Water
while the solids are generally referred to as
Mixed Wastes
The Recovery 2.0 system is based upon the FULL pyrolysis process designed to break materials down into basic elements,
as opposed to partial decomposition that only reduces waste into complex intermediate products.
Full or complete pyrolysis accommodates a wide variety of waste inputs irregardless of the calorific values and may
tolerate moisture contents up to 100%.
The input feedstock may be sourced from a wide variety of sources which will effect the content
or mix of raw materials contained within that particular waste stream. This will determine the blend of
product
outputs.
Output Products
Typical pyrolysis
outputs
are in the form of both gas & solids fractions.
The gaseous phase vapors that are produced are collected and processed in the
Hot Gas Refining
system.
The hot gas may be refined or condensed into a
liquid fraction.
The solid residues are extracted as a
solid fraction
for further processing.
Summary
Once the first stage of the thermal reduction process is complete, the feedstocks proceed to the intermediary stages of
Hot Gas Refining and
High Temperature Refining.
The materials are segregated into semi finished
output products.
The challenge is to design for optimum operation by
engineering a system that has the capability to swap from priority or default pathways
as seamlessly and rapidly as possible and the ability to scale up or down each energy pathway module.
Check-out
Recovery 2.0
