Metallic Fines & Oxides Recovery
Metal
Oxidation Fuels
may be one method that may be ideal for the of recovery of metallic fines and oxides is by applying
a series of
cascading
displacement reactions.
The
activation
of an Oxidation/Reduction & Displacement reaction is an energy efficient pathway to refine or purify
elements from various waste materials.
Implamentation of a Oxidation/Reduction (REDOX) recovery process may allow for the opportunity to extract energy from the reaction
and may be used in conjuntion with or as an alternative to
Hydrometallurgy.
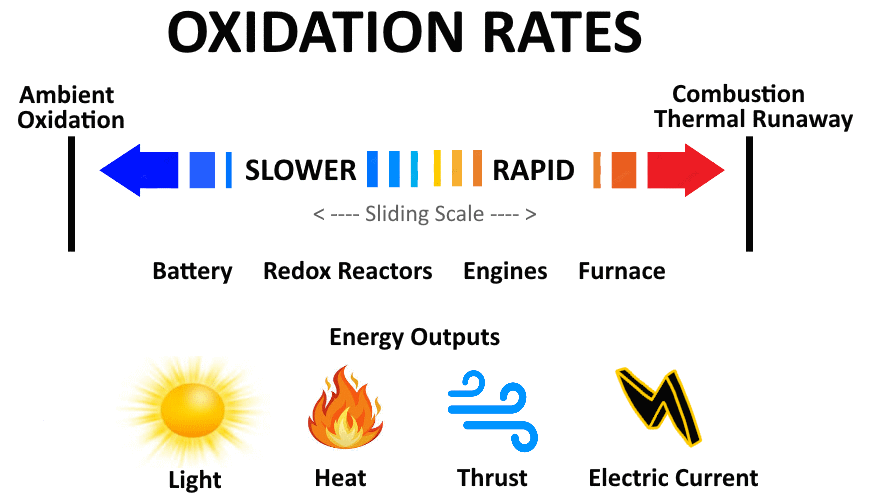
Oxidation Energy Rates
The development of methods or mechanisms with the ability to harvest energy from a full range of
the sliding scale of oxidation rates is a key focus of the
Recovery 2.0
strategy.
The Oxidation of a variety of materials including
Metal Fuels
generates a range of harvestable energy in the form of
Light - Heat - Pressure/Thrust - Electric Current
The speed and intensity of each of these types of energy determines the optimum approach to the harvesting methods.
Metal Oxide Recovery Options
Refined Metal Extraction -
Thermal Smelting
Oxidation/Reduction & Displacement Reactions
Cascading
sequence
of REDOX displacement
Exothermic Energy Extraction -
Metal Oxidation Fuels
Short Cycle Regeneration of Oxides
Harvesting Energy while Refining Metals

STAGE # 1. -
Copper Oxide Recovery
STAGE # 2. -
Indium &/or Tin Recovery
STAGE # 3. -
Iron Oxide Recovery
STAGE # 4. -
Zinc Oxide Recovery
STAGE # 5. -
Manganese Dioxide Recovery
STAGE # 6. -
Aluminum Oxide Recovery
STAGE # 7. -
Magnesium Oxidation Stage
STAGE # 8. -
Alkali Stage
Harvesting Energy
Energy Harvested as Heat or Converted to electricity
Multi-Stage Harvesting Points -
Incandescent Luminesce
May be converted to drive primary energy
source
Option to substitute an Alkali, Hydrogen or Carbon
(by default at any stage)